Background:
Daratumumab can provide deep hematologic responses for patients with light chain (AL) amyloidosis, both through its direct anti-clonal activity and immunomodulatory effects on other cell populations in the bone marrow (BM) microenvironment. Promising efficacy in the frontline setting when combined with CyBorD (per the ANDROMEDA trial), as well as in the relapsed/refractory setting as monotherapy, has led to daratumumab becoming the first FDA-approved therapy for AL amyloidosis. Though it is administered for up to 24 cycles, the optimal duration of treatment is unclear. Dynamic monitoring of measurable residual disease (MRD) may provide insights into changes in the aberrant plasma cell (PC) clone and sustainability of the hematologic response in relation to daratumumab therapy.
Aims:
We sought to evaluate the rates of MRD clearance and resurgence both during and after daratumumab therapy for AL amyloidosis.
Methods:
MRD status was assessed for patients followed at the Boston University Amyloidosis Center, who were treated with a daratumumab-based regimen and achieved a hematologic very good partial response (hemVGPR) or complete response (hemCR) according to consensus criteria (Palladini, 2012). MRD assessments were performed by multiparametric 10-color flow cytometry of BM aspirates (sensitivity level of ≤10 -5) and repeated at 12-month intervals during follow-up. Clonal progression was defined as growth in the aberrant PC clone size of >1-log.
Results:
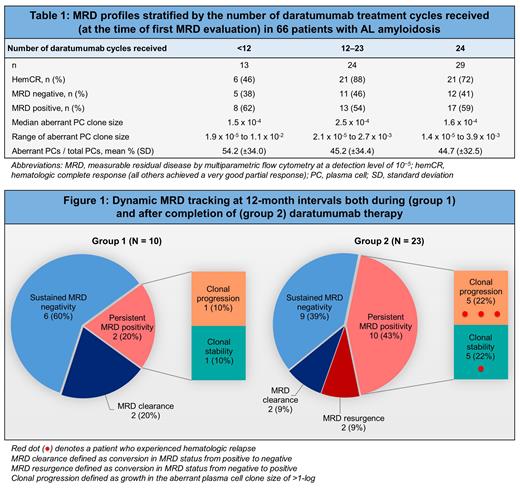
Between 2019 and 2023, a total of 66 patients achieved ≥hemVGPR and were tested for MRD; 43 (65%) received daratumumab monotherapy in the relapsed/refractory setting and 23 (35%) in combination with CyBorD as frontline therapy. Collectively, 48 (73%) achieved a hemCR and 18 (27%) a hemVGPR. The median age at diagnosis was 60 years (range 35-78); 19 (29%) patients were female; and 11 (17%) identified as non-white race. MRD assessment was performed once, twice or ≥3 times for 33, 18 and 15 patients, respectively. The first MRD evaluation occurred while still receiving daratumumab for 33 patients (at median 12 cycles, range 6-23); the remaining patients had their first MRD evaluation after therapy completion (median one month after, range 0-25). Of the entire cohort, 28 (42%) patients achieved MRD negativity and 38 (58%) MRD positivity (median estimated aberrant PC clone size 1.5 x 10 -4, range 1.4 x 10 -5 to 1.1 x 10 -2, representing 46.9% ± 32.8% of total PCs) at first evaluation. All but one patient with a hemVGPR had detectable MRD.
Of the 23 patients who received frontline daratumumab-CyBorD (18 hemCR and 5 hemVGPR), 8 (35%) achieved MRD negativity at first evaluation; whereas 20 (47%) of the 43 patients who received daratumumab as monotherapy (30 hemCR and 13 hemVGPR) achieved MRD negativity. We surveyed MRD profiles based on the number of daratumumab treatment cycles received (Table 1) and found no apparent patterns with increasing cycles.
Serial MRD monitoring was performed for 33 patients (Figure 1). Of 10 patients who continued to receive daratumumab between MRD assessments (group 1), 6 had sustained MRD negativity; 2 had persistent MRD positivity (of whom one had clonal progression); and 2 had MRD clearance (conversion from positive to negative). Of 23 patients who underwent serial MRD assessments after completing daratumumab (group 2), 9 (39%) had sustained MRD negativity; 10 (43%) had persistent MRD positivity; 2 (9%) had MRD clearance; and 2 (9%) had MRD resurgence (conversion from negative to positive), which occurred 17 and 26 months after daratumumab cessation. Clonal progression was observed in 5 of the 10 patients with persistent positivity, of whom 3 experienced a hematologic relapse.
Conclusions:
Our findings show that patients with AL amyloidosis can attain deep responses with daratumumab-based treatments, even to the depth of undetectable MRD. Clearance of MRD was frequent not only during treatment with daratumumab but also after completion of therapy, suggesting continuing effects on the plasma cell clone. Clonal progression after daratumumab completion was often associated with hematologic relapse. Prospective studies are needed to investigate whether daratumumab may be stopped early for those with undetectable MRD, or whether those with persistent detectable disease may benefit from extending daratumumab treatment beyond 24 cycles.
OffLabel Disclosure:
Szalat:Jansse Pharmaceuticals: Other: Participation to one advisory board. Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
This study includes off-label use of bortezomib for the treatment of AL amyloidosis.